
 |
|
 |
| Home | Research | People | Publications | News | Collaborations | Contact | Calendar |
| We
Focus Equally on the Pathogens ..... |
.... and on the Host Response to Infection | |||||||||||
| GAS |
GBS |
Staph |
Pneumo |
Anthrax |
S. iniae |
AMPs |
HIF-1 |
Glyco |
Macs |
NETs |
New
Rx |
|
 |
 |
 |
 |
 |
 |
|
 |
 |
 |
 |
 |
 |
| GAS
Molecular Pathogenesis |
Group
A
Streptococcus (GAS) is an important human pathogen
causing diseases ranging from simple pharyngitis ("strep
throat") to
invasive necrotizing fasciitis ("flesh-eating disease")
to the
immune-mediated syndrome of rheumatic fever. Our lab
aims to discover
and characterize GAS virulence factors by coupling
genetic approaches
(e.g. targeted mutagenesis) with tissue culture and
murine infection
models. Of particular interest are genetic mechanisms of
GAS innate
immune resistance and the shift from mucosal
colonization to systemic
disease. Among the GAS virulence factors we study are
the pore-forming
toxins streptolysins S and O (SLS and SLO), the
antiphagocytic and
proinflammatory surface M protein, DNAse Sda1, cysteine
protease SpeB,
hyaluronic acid capsule, serum opacity factor, IL-8
peptidase, and the
cell wall group A carbohydrate. Together, these studies
aim to provide
new targets for drug therapy and vaccine prophylaxis.
Our collaborators
include M. Walker (U. Queensland), P. Ghosh (UCSD), J.
Dixon (UCSD), M.
Kotb (Cincinnati) and E. Hanski (Jerusalem).
|
 |
|
| Group
B
Streptococcus (GBS) is the leading cause of invasive
bacterial infections in human newborn infants, including
pneumonia,
sepsis and meningitis. GBS is also increasingly associated
with severe
infections in nonpregnant adults, especially those with
underlying
diseases that weaken immunity. Our laboratory has studied
several
aspects of GBS pathogenesis through random transposon and
allelic
exchange mutagenesis paired with in
vitro cell culture models and
in vivo small animal challenges. Major areas of
investigation
have include the molecular genetics and virulence
properties of the GBS
pore-forming hemolysin/cytolysin toxin, functions of the
sialic
acid-expressing GBS polysaccharide capsule in molecular
mimicry and
evasion of innate immune clearance, mechanisms of lung
injury and
inflammatory responses in GBS pneumonia in the premature
infant, the
role of GBS surface proteins and cell wall components in
cellular
adherence and invasion, and the molecular basis of GBS
penetration of
and injury to the blood-brain barrier endothelium in the
pathogenesis
of newborn meningitis. Our collaborators include
Kelly Doran
(SDSU) and Ajit Varki (UCSD). |
GBS
Molecular Pathogenesis |
 |
| Staphylococcus aureus
Virulence |
Staphylococcus
aureus causes nosocomial and
community-acquired diseases including skin and soft
tissue infections,
osteomyelitis, bacteremia, abscesses, endocarditis and
septicemia.
Antibiotic resistance has reached epidemic proportions
in many regions,
with methicillin-resistant S. aureus
(MRSA) now exceeding HIV/AIDS as a cause of death in
the U.S. Our S.
aureus research includes study
of how the pathogen resists killing by human
phagocytes,
including the antioxidant properties of its
golden carotenoid
pigment, staphyloxanthin. Pigment inhibition, achieved
through
repurposing of human cholesterol-lowering agents, may
hold promise as
an adjunct to antibiotic therapy of MRSA.
Other immune
evasion factors under investigation include S. aureus nitric
oxide synthase,
phenol soluble modulins, Ig-binding protein A and the
pore-forming α-hemolysin. We are also
investigating S.
aureus
colonization and skin infection, to better understand
how the pathogen
both activates and resists cutaneous innate defenses. Our collaborators
include George
Liu (Cedars-Sinai), Eric Oldfield (U. Illinois), Pieter
Dorrestein
(UCSD), Suzan Roiijakkers (Utrecht) and AuricX
Pharmaceuticals
(Houston).
|
 |
|
| Streptococcus pneumoniae (SPN) is perhaps the leading cause of clinically significant bacterial infections worldwide, with a disease spectrum ranging from simple otitis media and sinusitis to invasive conditions including pneumonia, sepsis and meningitis. We have been interested in multiple roles of the surface-anchored pneumococcal neuraminidase (sialidase), NanA, in disease pathogenesis. In addition to its ability to cleave terminal sialic acid motifs on host cell targets, we have shown that an additional domain of the protein promotes invasion of brain microvascular endothelial cells and the development of pneumococal meningitis. Also, by cleaving sialic acid from host cell surfaces, the tonic engagement in cis of inhibitory Siglec receptors is released, leading to exaggerated leukocyte inflammatory responses. Finally, we have studied how NanactivitiesA sialidase modifies platelets and clotting factors to promote their clearance by the hepatic Ashwell receptor, influencing the development of disseminated intravascular coagulation during pneumococcal sepsis. Our collaborators include Ajit Varki (UCSD), Jamey Marth (SBMRI), and Kelly Doran (SDSU). | Pneumococcal Pathogenesis |
 |
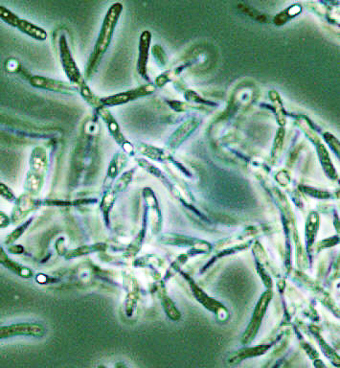
| Bacillus anthracis Pathogenesis |
Bacillus
anthracis
is a
Gram-positive spore-forming bacterium and the causative
agent of
anthrax. Primarily a disease of livestock, anthrax
can infect
humans through cutaneous, respiratory or
gastrointestinal routes of
infection. Inhalational anthrax occurs when
endospores are
introduced to the lung and taken up by resident
phagocytes -- the high
lethality of this disease makes anthrax a foremost
biodefense concern,
as evidenced by the 2001 postal attacks. We
are studying
novel functions of the anthrax toxins edema factor (EF)
and lethal
factor (LF) to inhibit endocytic recycling by the
Rab11/Sec15 exocyst,
leading to disruption of tight junctions and cell
barriers, a finding
we have generalized to other cAMP-inducing toxins. We are
also studying
the survival response of macrophages to LF inhibition of
p38 MAPK, a
pathway that involves activation of NOD2-dependent
inflammasomes via
ATP release and adenosine receptor signaling.
Finally, we are
looking at novel antimicrobial peptide resistance factors
such as the
ClpX protease which promote anthrax innate immune
resistance and
virulence.
Collaborators
include Ethan Bier (UCSD), Michael Karin (UCSD)
and Shauna
McGillivray (TCU).
|
 |
|
| Streptococcus iniae infections in aquaculture The controlled aquaculture of fish is an important and cost-effective industry for increasing the world's food supply. However, intensive aquaculture of several fish species has been threatened by infectious diseases, in particular a fatal meningoencephalitis produced by the ▀-hemolytic Streptococcus iniae. In recent years, we have conducted collaborative research project seeks to elucidate the virulence mechanisms of S. iniae using molecular techniques of random and targeted mutagenesis together with in vivo screening assays for virulence potential in hybrid-striped bass, tilapia and other species. Our goal is the rational development of effect vaccines and novel therapeutic strategies to protect aquacultured fish against this often devastating pathogen. A byproduct of this research has been participation in the discovery and characterization of novel fish antimicrobial peptides (e.g. moronecidin, bass hepcidin) and elucidation of their role in fish innate immune defense. | Streptococcus iniae in Aquaculture |
 |
| Cathelicidin AMPs in Skin
Immunity |
Cathelicidin
antimicrobial peptides (AMPs) In
a complex
environment, higher organisms face the constant threat of
microbial
infection. Effective first lines of defense against
infectious
pathogens comprise the innate immune system. A key
component of innate
immunity is the production of small, cationic AMPs, a
protection
strategy conserved from insects through man. In a
longstanding
collaboration with the laboratory of Richard Gallo (UCSD),
we have
adopted a combined mammalian and bacterial genetic
approach to decipher
the contributions of the cathelicidin family of AMPs to
host
immunity. Our studies with cathelicidin KO mice and
GAS infection
provided the first in
vivo
demonstration that endogenous expression of a mammalian
antimicrobial
peptide protects against invasive bacterial
infection. Ongoing
collaborative projects now explore additional
immunostimulatory
functions of the cathelicidin molecule, its
transcriptional regulation
by HIF and VitD in response to infectious challenge, the
molecular and phenotypic basis of bacterial
sensitivity or
resistance to AMP action, and the impact of bacterial AMP
resistance
on virulence and infectious disease epidemiology.
|
 |
|
| Hypoxia-inducible factor (HIF-1) Through a longstanding collaboration with the group of Randall Johnson (Cambridge and UCSD), we are examining the role of transcription factor HIF-1α as a key regulator of the bactericidal and inflammatory capacity of macrophages and neutrophils. Hypoxia is a characteristic feature of the tissue microenvironment during bacterial infection. HIF-1α is induced by infection, even under normoxia, and regulates the production of key immune effector molecules including granule proteases, antimicrobial peptides, nitric oxide and TNFα. Mice lacking HIF-1α in their myeloid cell lineage show decreased bactericidal activity and failed to restrict systemic spread of infection from an initial tissue focus. Conversely, activation of the HIF-1α pathway through vHL deletion or pharmacologic inducers supports myeloid cell production of defense factors and improved bactericidal capacity. Drug development for treatment of difficult infectious diseases is pursued in collaboration with Aerpio Therapeutics (Cincinnati). We are also examining the role of HIF-1α in modulating airway inflammation in asthma with L. Crotty Alexander (UCSD). | HIF-1 and Innate Immunity |
 |
| Host-Pathogen Glycobiology |
Glycobiology
of host-pathogen
interactions. The surface of all
bacterial and
human cells are covered with glycan molecules that play
a primary role
in arbitrating the outcome of the host-pathogen
encounter. We are
members of the UCSD Program in Excellence in
Glycosciences (PEG) and
Glycobiology Research and Training Center (GRTC)
spearheading
collaborative projects that examine the role of
bacterial glycans and
glycosidases in modulating myeloid cell innate immune
and inflammatory
responses. One major focus is the role of sialic
acid binding
lectins known as Siglecs in regulation of leukocyte
function, and their
subversion through molecular mimicry by GBS expressing
its sialylated
polysaccharide capsule. We are also studying the role of
GAS hyaluronic
acid in interactions with CD44, proinflammatory effects
of bacterial
sialidases and hyaluronidases, the role of host
glycosaminoglycans in
neutrophil and endothelial barrier function during
infection, and the
potential of reprogramming natural antibodies against
the nonhuman αGal
epitope to clear drug-resistant
pathogens via engineered RNA aptamers. Collaborators
include A. Varki
(UCSD), J. Esko (UCSD), R. Gallo (UCSD) and Altermune
Technologies
(London).
|
|
|
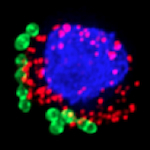
| Immune signaling in macrophages. In addition to our work with HIF-1 and Siglecs described above, we are probing a variety of signal transduction mechanisms and cellular pathways by which macrophages are rapidly activated in response to bacterial infection, but then able to resolve inflammation to limit collateral damage to host tissues. Areas of investigation include the roles of IKK/NFkB and MAP kinase pathways in regulating macrophage bactericidal and cytokine responses to infection, the function of NOD-like intracellular pattern receptors in response to bacterial toxins, mechanisms of inflammasome activation and IL-1▀ signaling, bacterial modulation of host cell apoptotic pathways, ATP/adenosine receptor signaling in neutrophil chemotaxis and bacterial killing, and the role of autophagy in host defense against intracellular pathogens. As a new participant of the NIH/NIAID Great Lakes Regional Center for Excellence in Biodefense and Emerging Infectious Disease Research, we are utilizing the information gained from these studies to design strategies for pharmacological enhancement of phagocytic cell function vs. antibiotic-resistant pathogens. Collaborators include Michael Karin (UCSD), Christopher Glass (UCSD) and Zev Ronai (SBMRI). | Immune Signaling in Macrophages |
 |
| Neutrophil Extracellular
Traps |
Neutrophil
extracellular
traps (ETs) consist of nuclear
(or
mitochondrial) DNA as a backbone with embedded
antimicrobial peptides,
histones, and cell-specific proteases providing a matrix
to entrap and
kill microbes . NETs
are formed after stimulation with mitogens, cytokines,
or pathogens
themselves, in a specialized cell death process
involving a
ROS-mediated signaling cascade and particular chromatin
modifications.
We are exploring roles of HIF-1α and cathelicidins in
the
generation of NETs at peripheral foci of infection, and
have uncovered
a novel contribution of the cholesterol biosynthetic
pathway in the
regulation of NET formation. We are also investigating
other immune
cells including mast cells and macrophages can
themselves produce
extracellular traps to control pathogens. Companion
projects examine
how specific bacterial factors (e.g. GAS M protein)
stimulate NET
production, whereas other virulence factors promote
bacterial
resistance to NET killing, e.g. by degradation of the
NET architecture
(DNAses of GAS and S.
aureus)
or resistance to the embedded cathelicidins.
Collaborators
include Maren von K÷ckritz-Blickwede (Hannover).
|
 |
|
| Novel antibiotic discovery. The continual emergence of antibiotic resistance among medically-important bacterial pathogens poses a great challenge to the public health. Sadly, the pipeline of new antibiotics in pharmaceutical development has yet to match this threat, with few novel antibiotic scaffolds developed in the last few decades. Our group is pursuing multiple parallel approaches for novel antibiotic discovery. These include evaluation of new chemical entities generated from marine actinomycete-derived natural product libraries, chemical genomic platforms, virtual screens, and medical chemistry modification of lead compounds. We also believe that outside-the-box approaches to infectious disease therapy including inhibition of virulence factors (e.g. S. aureus pigment) or pharmacological augmentation of phagocytic cell function (e.g. HIF-1α boosting) represent critical areas for exploration. Finally, through an NICHD-sponsored UCSD Research Program in Developmental Pharmacology, we are exploring synergy of pharmaceutical antibiotics with natural antimicrobial peptides, with a goal of optimizing therapy through innate immune sensitization. Collaborators include W. Fenical (SIO/UCSD), P. Dorrestein (UCSD), M. Burkart (UCSD) and E. Capparelli (UCSD) | Novel Antibiotic Discovery |
 |
|
Home
| Back to Top |